Abstract
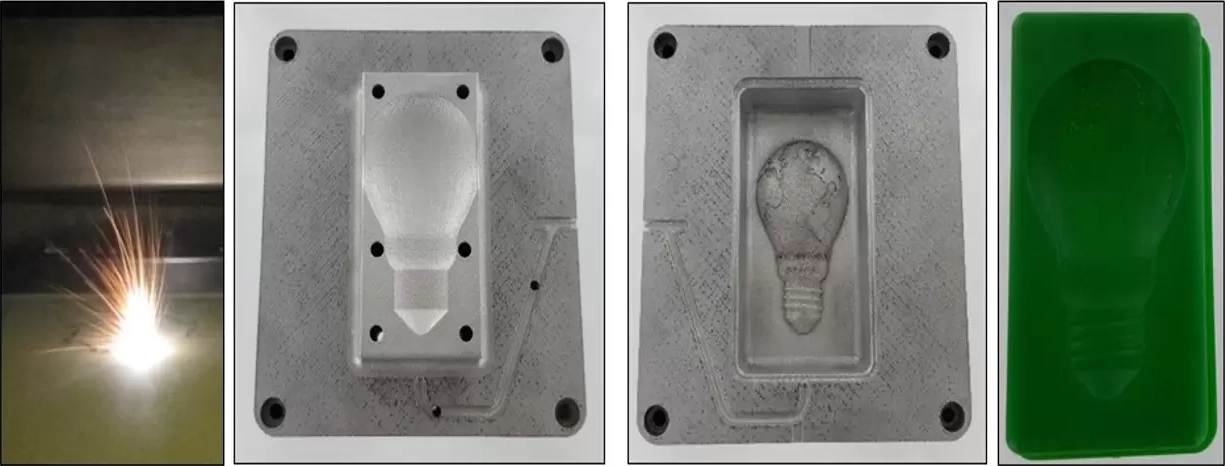
For the past 18-months, HARBEC has, along with project partners NYSERDA, Cornell, RIT, and Terrapin Bright Green, explored the nexus between how nature efficiently transfers heat and fluids, and applications for advancing thermal and fluid transfer within injection molds. Leveraging 3D printing, HARBEC and partners have demonstrated the potential for “growing molds” that incorporate principles of biomimicry, and resulting in more energy-efficient and higher-performance molds for manufacturing. Specifically:
- Principles of thermal and fluid transfer that are prevalent in nature, and which offer intelligent solutions for optimizing the transfer of heat and fluids in manufacturing tools.
- How additive manufacturing (AM) and 3D printing tools specifically, can incorporate principles of biomimicry into “grown molds” that enhance energy, productivity, and quality.